
It’s Easy to Get Started
 1
1
Select Your Mobile IV Treatment
Choose from any of our vitamin IV drips that best fits you or build your own treatment. 2
2
Book IV Therapy Appointment
Book your day and time that you prefer and one of our nurses will call to medically clear you.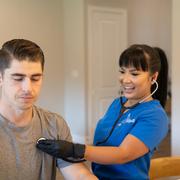 3
3
We Review Overall Wellness
Your IV Therapy case is reviewed by a certified healthcare professional. 4
4
Mobile IV Directly To Your Door
One of our Registered Nurses will come to the location of your choice to start the IV therapy. 5
5
Feel Better, Faster. We Come To You!
Sit back, relax and feel better with your customized mobile IV therapy treatment.
Choose the IV Drip for Your Symptoms
Each of our IV drip treatments is specially crafted by our pharmacist and chock full of fluids, antioxidants, vitamins, medicines, and nutrients to help tackle a number of common symptoms people experience when they aren’t feeling 100%.
Best of all, we come to you for at-home IV fluids! The result of our IV hydration therapy packages — you end up feeling great. Our exclusive Mobile IV Medics (MIVM) Cocktail is our bestseller. This all-inclusive IV package gives you some of everything we offer and takes care of all your hydration needs.
We have a variety of IV drips to help alleviate an array of symptoms and conditions such as migraines, dehydration, hangover, and chronic pain, or you can create your own custom IV drip.
Our Mobile IV Treatments
Select Any Of Our Most Popular IV Drips
Create Your Own Vitamin IV Bag
Vitamin IV Bags Start At $199. Additional Add-Ins Are +$20.Vitamin B12
A powerhouse for the body, helping make DNA, nerve and blood cells. Your metabolism cannot function without it and no metabolism, no energy
Vitamin C
Vitamin C helps with many aspects of your health including anti-oxidant effects and neutralizing free radicals, boosts in energy, mental clarity, and memory, promotes healthy skin and skin structure, and a potent immune system.
Vitamin B Complex
Supplementation of B vitamins has been shown to improve ratings of stress, mental health, vigor, and cognitive performance and works well with infusion therapy and overall wellness.
Glutathione
Your body’s most powerful antioxidant and detoxifying agent. It’s essential to eliminating toxins, maintaining your youth, and sustaining your wellness.
Taurine
It is an essential amino acid for neurotransmission, neuroprotection, and neurogenesis. Taurine is beneficial in regulating your mood and stress levels to help you focus and devote your energy to the tasks at hand.
Magnesium
Vital Nutrients are important, and magnesium is no exception. It participates in many important bodily functions including energy creation, protein formation, genetic maintenance, muscle function, and nervous system regulation for overall health.
Zinc
Helps heal, protect, and rejuvenate your skin. It may reduce the duration of your cold by half and has some direct effects on your bug and may slow the reproduction of microbes.
Anti-Nausea Medicine
This works directly in the areas of the body to block the transmission of signals in your nervous system that have caused your upset stomach. This medicine is very effective so take solace in knowing your nausea and vomiting should end quickly.
Anti-Inflammatory Medicine
Need something to take care of your aches, fever, headache, and/or generalized pain and do so quickly, all while replenishing your body’s fluids? That is exactly what this anti-inflammatory medication will do.
Anti-Heartburn Medicine
This medicine will help temporarily decrease the amount of acid secreted in your stomach, allowing you to better tolerate the duration of your illness and decrease your nausea.

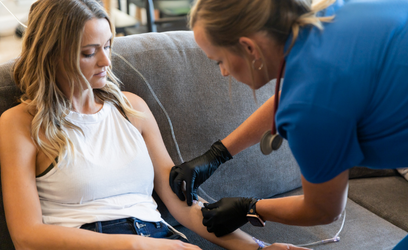
IV Therapy 101
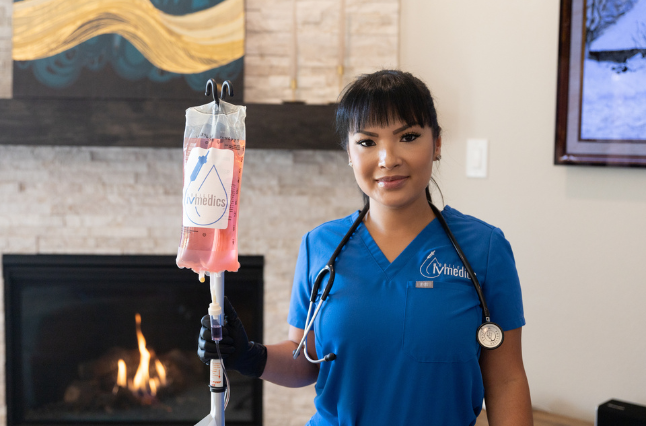
When you think of an IV drip, you probably picture a trip to the hospital or ER. A needle is inserted into a patient’s arm to deliver treatment when someone is sick. But IV Infusion Therapy can be used for so much more. At Mobile IV Medics, we offer specialized IV Therapy services delivered in the comfort of your home, office, or hotel room making it a truly convenient way to feel better faster.
IV Therapy is the fastest way to get your body the vitamins and fluids it needs to help alleviate the uncomfortable symptoms associated with dehydration. The intravenous route is the fastest delivery method because it introduces the benefits of the IV drip directly to your circulation, replacing fluids, nutrients, and vitamins throughout your body.
An IV drip can quickly and effectively alleviate symptoms like migraines, nausea, and many more, speeding up your recovery from hangovers, colds, flu, food poisoning, and other conditions. Vitamin IV drips can also be used to help improve your overall wellness, leaving you feeling energized and just all-around good.
A visit to the emergency room to address the effects of the flu, food poisoning, or hangover takes a lot of time out of your day and can end up being a costly hassle. You don’t need to search for “IV Bar near me” either. We come to you to save you time and energy offering customizable IV therapy solutions to address your specific symptoms. You can choose from our list of vitamin and nutrient add-ins to create your custom IV bag based on your symptoms. Or check out our pharmacist-crafted selections. Before long, one of our Registered Nurses will be by your side to administer the infusion. It’s that simple.
About Mobile IV Medics
Save your time and energy, let the Mobile IV Medics team bring our concierge IV therapy services to you.
As a mobile IV therapy service throughout California, Colorado, Florida, Georgia, Illinois, Nevada, Pennsylvania, Tennessee, and Texas our team of healthcare experts and professionals will treat you as soon as possible without having to leave the comfort of your home, hotel, or office.
Whether you’re dehydrated, debilitated from a migraine, hungover, jet-lagged, suffering from a cold or flu, looking for wellness and beauty, recovering, or just have low energy, our IV treatments of fluids, vitamins, minerals, and medicines will provide you with the care you need!



MOBILE IV MEDICS REVIEWS
What People Are Saying
Chicago, ILBetrice was awesome. Would definitely sign up again.
Los AngelesIncredible, professional, and clean service! So thankful I found them. I felt AMAZING and so much energy after my IV. Definitely going to be an IV drip addict after my first session.
Santa MonicaI had one of the best experiences of my life in the hands of Richie! He took a very consultative approach to which particular cocktail suited my symptoms and I felt better almost immediately. He was personable, cordial and very meticulous about sterilizing during COVID. I would highly recommend to anyone – I know I will be coming back and asking for Richie again!
Santa BarbaraNurse Richie is incredible and changed my life! I tend to get bad hangovers and have always wanted to get an IV. I was in Santa Barbara for my wedding week and felt horrible one morning.
We called Nurse Rich’s and he was incredible: professional, knowledgeable, and meticulous. Beyond that he is such a kinda person who was incredibly accommodating to my friends and myself.
We loved him and the service so much we ended up coordinating with him to get IVs for my bridal party!
If you get bad hangovers you don’t have to suffer anymore – and there is no one else I would trust more than Nurse Richie.
Santa BarbaraRichie was so nice and friendly! Ordering an IV was a quick and easy process and they were at my house within the hour. I didn’t know what to expect but it was a great experience! The hangover IV helped me turn the corner and feel so much better. HIGHLY recommend.
Santa BarbaraRichie was a life saver!! I ended up getting the flu for my wedding and he came the night before. I was able to sleep through the night. My best friend messaged him and asked if he was able to come the next day. He didn’t have any openings but went out of his way to help me and fit me in. And oh did it help!! I felt so hydrated and energetic. Thanks to him I actually enjoyed my wedding! He’s the best!
Santa BarbaraAfter returning from a trip yesterday I was feeling tired and like I may be coming down with something. Messaged IV Medics and call it kismet, fate or the stars aligning they were just finishing up down the street and could be at my house in 10 minutes. The nurse went through all the IV options thoroughly as well as answered all my questions. My daughter decided to join me since the set up was quick & painless AND she could continue working. IV Medics took the time to follow up with us tonight and we were both thrilled to report that we were feeling great! We both are looking forward to many more appts in the future for the whole family with IV Medics.
Santa BarbaraThe Best! Professional, knowledgeable, kind and thorough – Nurse Richie is one of the top IV infusion and home nurse visits ever! (did you know he has his masters in nursing?!)
We were so lucky he managed to get us in same day.
An intuitive bedside manner as well. Highly recommend Nurse Richie – the reason IV medics really stands out above the rest
Santa BarbaraRichie is such a gift! I got my second Covid vaccine today and was feeling sluggish. I’ve also just been majorly stressed out lately. My best friend who lives up north purchased the hydration iv for me to help with everything. A major thank you to my BF because Richie was just what I needed today. He was super fast to respond and let me know when he was on his way. He was professional but also felt like a long time friend! He is funny, smart and knows his stuff! He explained everything to me helped me understand the benefits of what vitamins would be useful. And honestly, he brightened my mood because he is just such a joy to be around 🙂
I already booked 4 more sessions with him. I couldn’t be happier. I feel much better as I mentioned earlier that I was feeling sluggish. He checked in with me later to see how I was feeling. He truly cares and it shows.
Santa BarbaraI wish Richie was available in San Francisco.
Anyone who is in need of hangover relief in the Santa Barbra area needs to immediately contact Mobile IV. Came to our hotel within a hour and Richie was personable and made the experience as comfortable as possible.
Life changing.

Schedule An IV Drip
"*" indicates required fields











